Our product team combines a wealth of expertise in product design, engineering, and manufacturing. We undertake product design projects from initial specification, sketching and CAD designs right through to prototyping, testing, validation, certification, and manufacturing. We understand exactly how to get your medical device idea ready for market. We approach each project with the same philosophy to ensure that we understand our clients’ needs, then develop bespoke solutions around their specific requirements. We do more than create the design - we will support you through every step of the process including support and advice on achieving the necessary regulatory approvals. We deliver products which are robust, safe and perform well under the rigours of anticipated use.
Not only will we create high quality prototypes that will allow you to showcase your product we can also develop, validate, and facilitate the production of the chosen packaging.
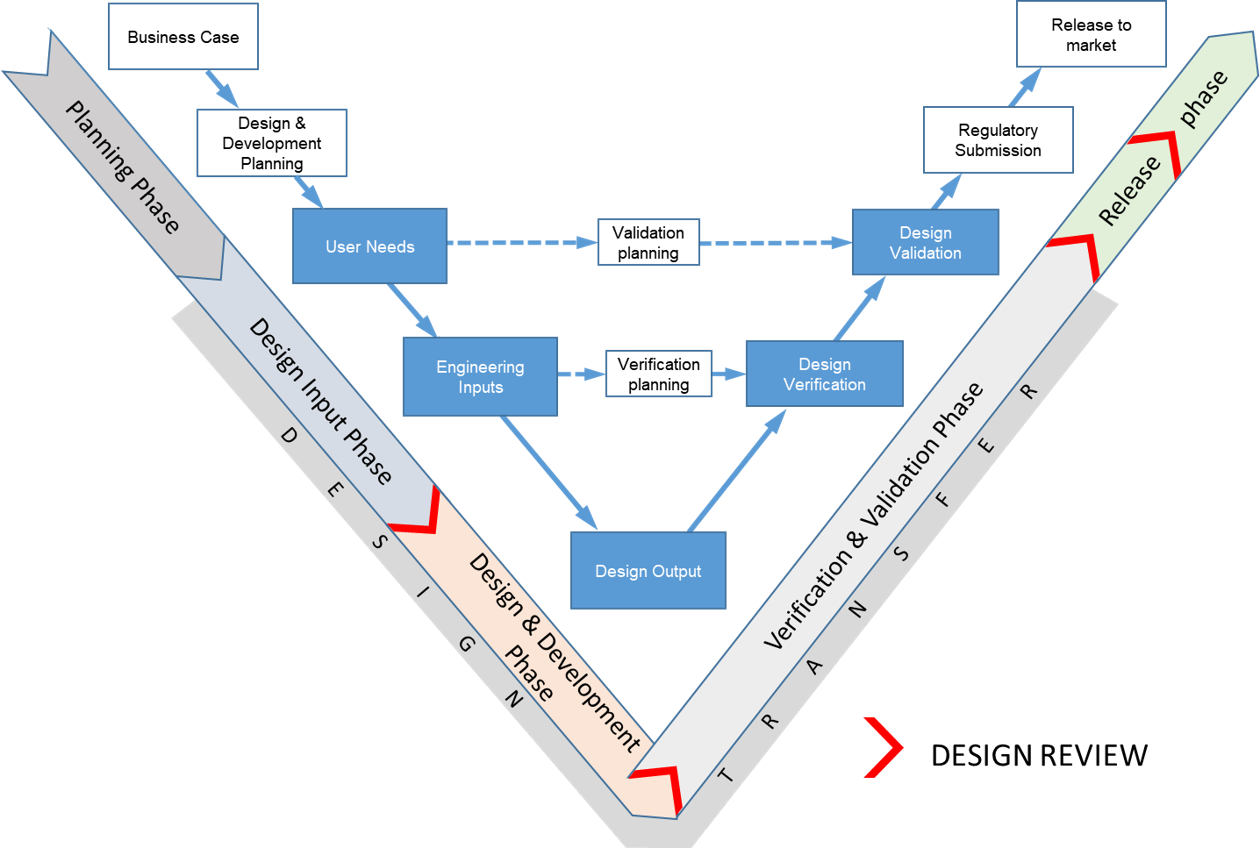
OxDevice Design Controls Overview
OxDevice operates a quality system in accordance with EN ISO 13485:2016 and FDA QSR 21CFR820. Design and development activities are managed in accordance with these requirements and include the following:
- Design planning
- Design inputs
- Design outputs
- Design review
- Design verification
- Design validation
- Design transfer
- Control of design and development changes
- Creation and maintenance of design and development files